
straight to your inbox
Conducting pharmaceutical research and clinical trials is arduous, intricate, and considerably costly. Navigating the intricate financial ecosystems involved in bringing a new drug or treatment to market is an essential skill set for researchers, clinical trial managers, and finance teams. Effective expense management in this field is more than just accounting for monetary transactions; it's a strategic approach to ensuring the most efficient use of resources while maintaining compliance and meeting the high standards of research integrity mandated by regulatory bodies. This guide will walk you through the meticulous steps required for successful management of expenses in pharmaceutical research, offering a comprehensive toolkit you can employ to minimize financial risks and maximize the potential of your endeavors.
Step 1: Establish Budget Guidelines
Setting the right budget at the start of your research or trial is foundational to financial control in the long run. To achieve this:
Define Budget Objectives and Constraints
Be clear on what you aim to achieve with research and the constraints you face. These could include time constraints, funding limitations, and any specific requirements for the trial.
Allocate Funds for Different Research Phases
Break down funding requirements by distinct phases of research or trial. This provides clarity on which stage commands how much of your overall budget.
Step 2: Track Expenses in Real-Time
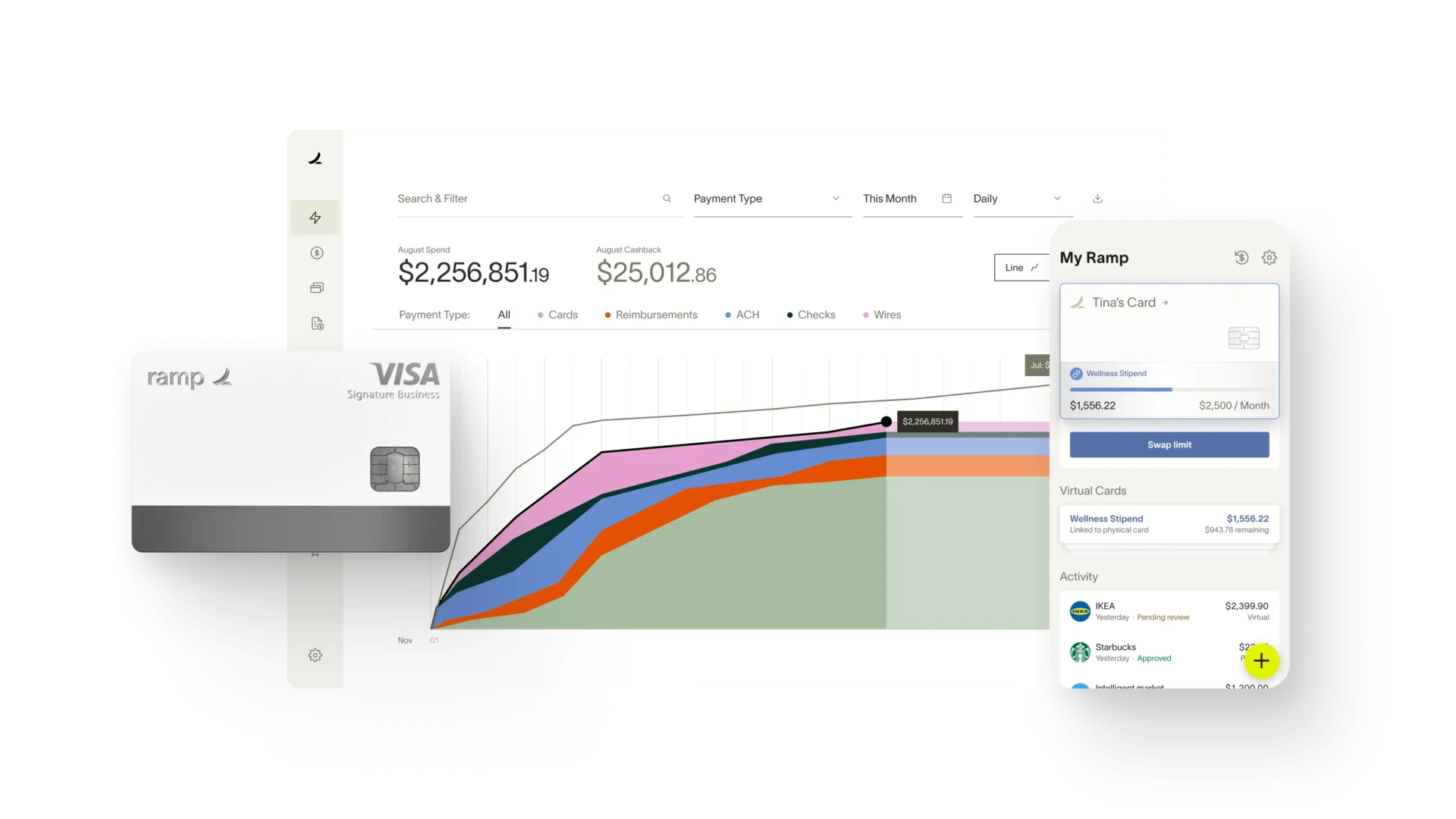
Accurate, up-to-date expense tracking is a pillar of good financial management.
Utilize Expense Tracking Software or Spreadsheets
Use tools that align with the scale and complexity of your research. Modern software can offer real-time data and ease of use, while spreadsheets provide versatility for smaller teams.
Categorize Expenses by Research Activity or Trial Phase
This enables more precise tracking and provides insight into which activities are consuming the most resources.
Step 3: Monitor and Control Costs
Overspending can be detrimental to the integrity of your research and future viability of your projects. Take control with regular monitoring.
Regularly Review Expenses Against Budget
Set a schedule for financial reviews to ensure you stay on track and detect any discrepancies as soon as possible.
Identify Areas of Overspending or Cost-Saving Opportunities
Regular financial check-ins reveal patterns and give you the opportunity to make informed, real-time decisions that can optimize your spending.
Step 4: Streamline Expense Approval Process
An efficient approval process saves time and reduces the risk of delays that can lead to cost overruns.
Implement Clear Approval Workflows
Everyone involved should know their role in the approval process. Document it clearly to prevent confusion and bottlenecks.
Minimize Delays and Bottlenecks
Use technology and proactive management to ensure approvals are given swiftly, with minimal delays.
Step 5: Maintain Accurate Documentation
Complete and detailed records are mandatory for accounting transparency and regulatory compliance.
Keep Detailed Records of All Expenses
Document every transaction with date, amount, and purpose, accompanied by any necessary approvals.
Ensure Compliance With Regulatory Requirements
Regulatory bodies often require financial transparency for clinical trials. Keeping ahead of these requirements saves time and mitigates risks.
Step 6: Analyze and Optimize Expense Patterns
Regular analysis of spending patterns allows for course correction and improved efficiency.
Use Data Analytics to Identify Spending Patterns
Modern expense management tools can provide insights into historical data that may reveal hidden patterns and cost drivers.
Adjust Budget Allocations and Strategies Accordingly
Data-driven decisions facilitate the agility necessary to optimize spending across the different stages of your pharmaceutical research projects.
Effective expense management in pharmaceutical research and clinical trials is essential for success. It ensures that the focus remains on research excellence while being financially responsible. By carefully budgeting and tracking expenses, maintaining clear documentation, and analyzing spending patterns, you pave the way for more efficient and fruitful research outcomes. Expanding your knowledge of financial management will not only bolster the current state of your projects but also set a robust foundation for the intricate financial journey awaiting in the pharmaceutical space. Remember, the key to mastering the art of managing research expenses lies in vigilance, adaptability, and a constant drive for improvement.










